When we buy a product or use a service, we essentially “hire” it to help us do a job. This is illustrated in the everyday consumer world by businesspeople hiring a milkshake in the morning to do the job of “providing a tidy way to satiate their hunger during long commutes” [1] or by people hiring iPhones “to check stock portfolios while they’re waiting in a line.” If you understand the outcomes people desire around their core Jobs-to-be-Done, you can create solutions that they’ll want to buy as we describe in our recent article.
But pharmaceutical and healthcare industries tend to be blinded by intense glare from shiny new scientific breakthroughs, enabling technology platforms, or legal/regulatory rumblings. These aspects surely describe the landscape in which drugs are discovered and healthcare is delivered, but they have little to do with the actual jobs-to-be-done by job executors along the path from drug discovery to patient care. This leads to a “solutions in search of problems” mentality as we describe in another recent article. Worse, it leads to a gap between healthcare expenditures and outcomes that has reached an unsustainable level. Lacking a “true North” provided by singular focus on a few, core Jobs-to-be-Done, innovations in biopharma are generally competing against luck. [2]
In this article, we provide three concrete examples of core Jobs-to-be-Done in biopharma that provide fresh perspectives for financing, innovating, and marketing solutions in the healthcare therapeutics/diagnostics world.
“Jobs-to-be-Done Theory unlocks the mystery that has for decades been clouding the understanding of customer needs. Knowing how to classify all customer needs changes everything.” – Anthony Ulwick [3]
Pharmaceutical Drug Discovery
A core Job-to-be-Done in pharmaceutical discovery is to “select the next group of compounds to synthesize in order to test a hypothesis at a given stage of a drug discovery project.” This then defines a market: medicinal chemists and other project team members (e.g., computational chemists) who need to select the next group of compounds to synthesize in order to test a hypothesis at a given stage of a drug discovery project [4].
Desired outcomes related to this job include the following:
- Minimize the time required to select the group of compounds
- Minimize the time to synthesize (or acquire) selected compounds
- Maximize information content (Shannon Entropy) of the results of the tests for the compounds that were selected (i.e., you’ve learned something important from having performed the test)
- Maximize the likelihood that the compounds will be novel – yet not considered obvious extensions of existing patented compounds
- Maximize the likelihood that compounds will bind more tightly to selected targets than the previous set of tested compounds – without sacrificing selectivity
- Maximize the solubility of the compounds (so they can be accurately assayed)
- Maximize the likelihood that compounds will exhibit good PKPD properties
- Maximize the likelihood that the project team will reach a true consensus on which compounds are selected
- Minimize the costs to synthesize and assay the selected group of compounds
The Job-to-be-Done in this example and all desired outcomes are gathered qualitatively through ethnological observation or interviews [5,6], while recognizing that this core job and its associated desired outcomes have not just functional – but also emotional and financial – components. While the first seven desired outcomes relate to the execution of the core job, outcome #8 relates to an emotional job and outcome #9 relates to a financial job. In most cases, there will be 50 to 100 desired outcomes in total associated with a given job.
Note that these desired outcomes (needs) are specifically phrased to be solution agnostic. In Figure 1 (below), people in the blue segment struggled with desired outcomes 2, 6, and 9 (Set i) to get the job done, while the people in the red segment struggled with outcomes 4 and 5 (Set j). In this depiction, people represented by green circles successfully achieved the desired outcomes sets i and j after doing the job of “selecting the next group of compounds to synthesize in order to test a hypothesis at a given stage of a drug discovery project.”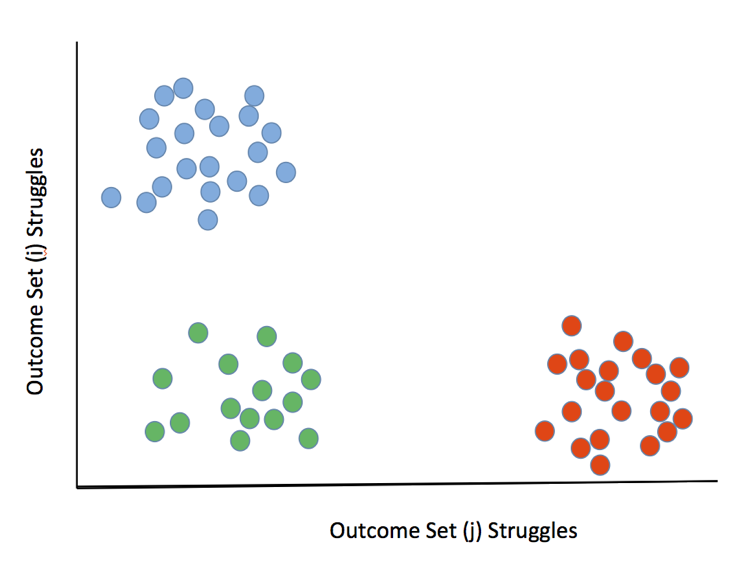 Figure 1. Market segmentation by clustering people who are uniquely struggling around sets of desired outcomes (i.e. unmet needs) around the core Job-to-be-Done
Figure 1. Market segmentation by clustering people who are uniquely struggling around sets of desired outcomes (i.e. unmet needs) around the core Job-to-be-Done
Drug discovery teams in the blue segment know where they’re headed, so solutions to their problem set would have features focused on operational efficiency. Alternatively, teams in the red segment are struggling in scientific areas, so solutions to their problem set might involve software that allowed one to robustly predict whether binding affinity of a given compound to a given target would be expected to be higher or lower than that of previous compounds.
Clinical Trials
One core Job-to-be-Done for Contract Research Organizations (CRO’s) is to “generate data to make go/no-go decisions to move forward to the next phase of clinical development.” Inherent in this job statement is the notion that sponsor organizations want to get the “signal” well before submissions to the FDA. For small biopharma, a related job to this may be to “generate data to attain value inflection in the eyes of a sponsor’s investors.”
This then defines a market: clinical researchers and other project team members (e.g., project managers) who need to “generate data to make go/no-go decisions to move forward to the next phase of clinical development.”
Desired outcomes related to this job include the following:
- Minimize the number of patients needed to be enrolled in the trial
- Minimize the amount of endpoint data collected to reach a decision point
- Minimize the time needed to reach a decision point
- Maximize the likelihood that the trial will have sufficient statistical power
- Maximize the likelihood that the effect size will be clinically significant
- Maximize the likelihood that positive results from Phase N will be predictive for positive results in Phase N+1
…
- Minimize the time needed to enroll patients in the study
- Minimize the complexity of the study design
After performing clustering as depicted in Figure 1 (above), a given segment of clinical researchers would be selected and their struggles with each individual desired outcome would be analyzed in more detail.
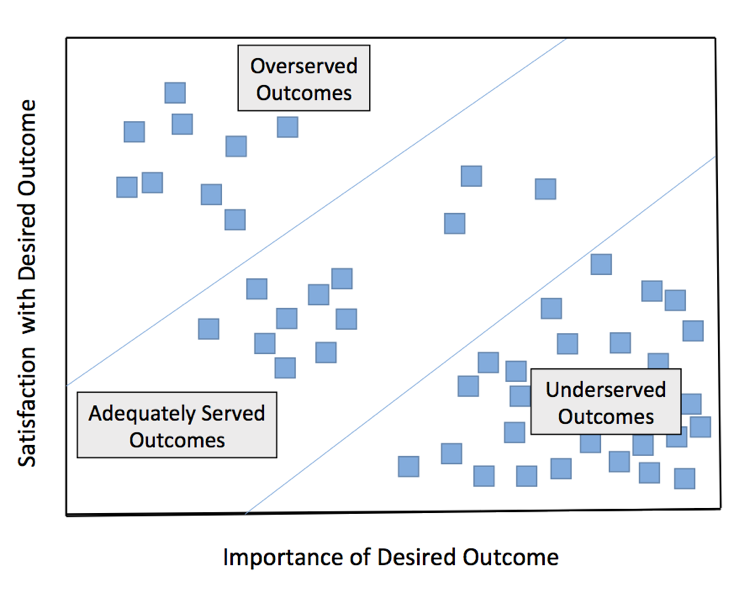 Figure 2. Analysis of Outcomes Associated with a Selected Market Segment
Figure 2. Analysis of Outcomes Associated with a Selected Market Segment
Let’s assume that we identified a cluster of clinical researchers (using mapping similar to that in Figure 1) whose underserved desired outcomes for the Job-to-be-Done mainly included outcomes 1-6 above (depicted in the bottom right triangular region in Figure 2). This would furnish a template for a solution where CRO’s offered NGS-driven, genomically stratified trial designs with molecularly defined inclusion and exclusion criteria, which would increase the chances of observing large effect sizes to reduce sample requirements, increase statistical power, and increase predictive power (i.e. satisfy unmet desired outcomes 1-6).
Patient Treatment
One core Job-to-be-Done by physicians treating cancer patients is to “select treatment for patients who relapse after resistance to front-line therapy from tumor re-emergence that has a different genetic architecture than the original clone.”
This then defines a market: specialized molecular tumor boards (consisting of oncologists, radiologists, pathologists, geneticists, surgical oncologists, bioinformaticians, etc.) who need to “select new treatment for patients who relapse after resistance to front-line therapy from tumor re-emergence that has a different genetic architecture than the original clone.” A related job to this core job would be to “detect disease relapse after successful primary treatment.”
Desired outcomes related to this core job include the following:
- Maximize sensitivity for genetic mutations in known hot spots
- Maximize relevant clinical, radiological, and omics patient data available for review
- Minimize the time required to aggregate this data
- Maximize the likelihood that either an approved drug or a current clinical trial will be available to a given patient whose case is under review
- Maximize the likelihood that the selected second-line treatment will have a long progression-free survival
- Maximize the likelihood that the Tumor Board would achieve a consensus
- Minimize the likelihood that surgeons and radiologists would feel overwhelmed by the depth and scope of the genomic/transcriptomic information presented by geneticists and bioinformaticians
Note that this is a very different job from that of “screening a healthy population for early detection of cancer,” which – in turn – defines a different market. And it is also a different market from that of lone oncologists who need to “select new treatment for patients who relapse after resistance to front-line therapy from tumor re-emergence that has a different genetic architecture than original clone.” In the latter case, the genomic information would need to be distilled in a way that was consumable by a non-expert.
From a solution-side perspective, liquid biopsy technologies have altered the landscape by allowing treatment teams to make actionable decisions at each stage of a patient’s journey through the cancer life cycle. However, (i) early detection, (ii) monitoring of initial treatment, and (iii) creation of a personalized treatment plan if and when relapse occurs (the Job-to-be-Done in our example above) are actually very different jobs (and hence markets), which have different executors, different desired outcomes, and take place in different points in space and time. The conflation of jobs/markets i and ii with our defined market iii would lead to a “one-size-fits-all” approach, where none of the liquid biopsy offerings would contribute to an optimized solution for any given job. Moreover, the emphasis on the technology – instead of the situation in which a physician needs to accomplish a task (i.e., core job) – forces practitioners to cobble together solutions from various vendors to get a job done.
For example, there are liquid biopsy offerings focused on highly targeted hot spot genomic mutations, while there are others that feature wider – but less deep – coverage. In the informatics part of the Job-to-be-Done, there are solutions for aggregating omics data – such as genomics, transcriptomics, epigenomics, proteomics, and metabolomics – that solve part of the job. But there are others that purport to aggregate omics data with EHR and imaging data, which – in principle – address the job more comprehensively. The right approach depends on the Job-to-be-Done and market segment you decide to make your singular focus.
Recommendation: Gather the Data You Really Need
Jobs-to-be-Done theory [1,2], Outcome Driven Innovation [3], and Voice of the Customer [5,6] have been widely recognized for two decades – yet very little of this has trickled down into the biopharmaceutical/diagnostics field. That’s because the Venn diagram overlap between people having the methodological expertise in these needs-gathering platforms, the requisite scientific domain expertise, and the time/permission to carry out these studies is practically nil.
Do yourself a favor: Set up a consultation with a business advisory that can quickly and cleanly gather the only data that your company really needs – and will be done with minimal disruption and maximum impact.
[1] Christensen, C. M., and Raynor, M. E. (2003). The Innovator’s Solution: Creating and Sustaining Successful Growth. Harvard Business School Press.
[2] Christensen, C. M., Dillon, K., Hall, T., and Duncan, D. S. (2016). Competing Against Luck: The Story of Innovation and Customer Choice. Harper Business.
[3] Ulwick, T. (2016). Jobs to be Done. Idea Bite Press.
[4] Maynard, A.T., Roberts, C.D. (2016). Quantifying, Visualizing, and Monitoring Lead Optimization, J. Med. Chem. 59 (9), 4189-4201.
[5] Griffin, A. & Hauser, J. (Winter 1993). The Voice of the Customer, Marketing Science 12(1), 1-27.
[6] Katz, Gerald M. (2004). Chapter 7, The Voice of the Customer. The PDMA Toolbook for New Product Development (p. 170). John Wiley & Sons.
